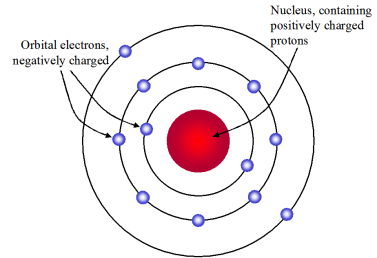
Rutherfords model shows that an atom is mostly empty space with electrons orbiting a fixed positively charged nucleus in set predictable paths. Rutherfords atomic theory proposed a dense nucleus surrounded by very small electrons.

Explain Rutherford S Model Of Atom Brainly In
Solution aRutherfords model shows that an atom is mostly empty space with electrons orbiting a fixed positively charged nucleus in set predictable paths.
. Almost the entire mass of an atom lies in the nucleus. Rutherford used αparticle scattering experiment on a gold sheet. Rutherfords model of atom can be described as follows.
This was based upon the bright-line spectra of molecular hydrogen and lead to postulates that describe the electronic structure of the atom as having electrons in discrete energy levels and orbiting the nucleus much like planets orbit a star. Rutherfords Nuclear Model of Atom. Rutherfords atomic model is the model which described the atom as a tiny dense positively charged core called a nucleus in which nearly all the mass is concentrated around which the light negative constituents called electrons circulate at some distance much like planets revolving around the Sun.
Rutherfords Model of the Atom Rutherford proposed that each atom has a dense central core which he called the nucleus The nucleus is a central region that is very small relative to the total size of an atom The nucleus contains virtually all of the mass and all the positive charge of the atom Problem- the electron orbits as some distance. 21 hours agoTo assist describe this Bohr recommended that the feasible orbit s in a hydrogen atom rise by n 2 where n is the primary quantum number According to Bohrs design a covering 3 to shell 2 shift creates the initial line of the Balmer collection For hydrogen this makes a photon having a wavelength of 656 nm or traffic signal as seen. In the nuclear atom the protons and neutrons which comprise nearly all of the mass of the atom are located in the nucleus at the center of the atom.
If all matter is mainly empty space why is it impossible to walk through walls of pass your hand through your desk. Thomsons had electrons moving through a sea of positive charge sometimes called the plum pudding. 3 Atoms nucleus is surrounded by negatively charged particles called electrons.
Plum Pudding Model Rutherfords Model. How does this model explain the deflections of a beam of alpha particles aimed at a sheet of gold foil. Which of the following statements BEST describes the location of electrons in Rutherfords model of the atom.
Describe Rutherfords model of the atom including the location of protons neutrons and electrons with respect to the nucleus. Three rules tell us how. A Rutherfords model of atom- Ernest Rutherford was interested in knowing how the electrons are arranged within an atom.
He conducted an experiment in which a thin gold foil was bombarded with fast moving alpha α particles. He observed that most of the α particles passed through the gold foil without deflecting. He selected a gold foil because he wanted a layer as thin as possible.
1 Atoms have their charge concentrated in a very small nucleus. Rutherfords Atomic Model. An atom consists of a positively charged dense and very small nucleus containing all the protons and neutrons.
Rutherfords atomic model became known as the nuclear model. Electrons are both inside and outside of the nucleus. Describe rutherfords model of the atom.
Chapter 5 Electrons in Atomsare the way electrons are arranged in various orbitals around the nuclei of atoms. The electrons are distributed around the nucleus and occupy most of the volume of the atom. According to Rutherford model of the atom.
This model of an atom was developed by Ernest. Chemistry 08102019 0800 dontcareanyonemo. The gold foil was about 1000 atoms thick.
Rutherfords famous α-particle scattering experiment is represented in Figure. 2 Major space in an atom is empty. Rutherford and his students Hans Geiger and Ernest Marsden bombarded very thin gold foil with α-particles.
Rutherfords model had a positive nucleus at the centre of the atom surrounded by electrons. Drawbacks of Rutherford Atomic Model As before Rutherford atomic model was also challenged and questioned by many. Previous question Next question.
Some αparticles were deflected by small angles and some were deflected by. Aufbau principle - electrons enter the lowest energy. This implies that atoms are composed mainly of empty space.
A stream of high energy a-particles from a radioactive source was directed at a thin foil thickness 100 nm of gold metal. The Rutherford model was eventually replaced by the Bohr Concentric Ring Model of the atom. The electrons are inside the nucleus.
This model of an atom was developed by Ernest Rutherford. The electrons are outside the nucleus. Models Of The Atomexplain how an atom can emit light or the chemical properties of an atom.
There are no electrons in the Rutherford Model.

Rutherford S Atomic Model Youtube

Rutherford Atomic Model Observations And Limitations In Detail

Rutherford Model Which Best Describes Rutherford S Model Chemistry


0 Comments